Understanding the Mechanisms Behind Radioisotope Analysis
Written on
Chapter 1: Introduction to Radioisotope Dating
In discussions with proponents of creationism and various pseudoscientific theories, I frequently encounter claims such as, “Radiocarbon dating is incredibly inaccurate; it misjudges the age of objects,” or “Radiocarbon dating is irrelevant for items older than 5, 10, or even 20 thousand years; thus, scientists cannot accurately date fossils, leading to a fundamental deception about the age of fossils and the Earth itself.” While these assertions contain some truth, they also reflect a significant misunderstanding of the methodologies used for age estimation. Let us delve into the workings of radioisotope dating together.

Chapter 1.1: The Basics of Radioisotope Dating
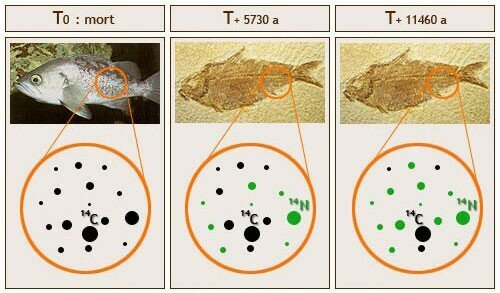
Radioisotope dating encompasses a variety of techniques, each relying on specific chemical elements and having distinct applications. The underlying principle across all forms is the law of radioactive decay. When any object is formed—be it a plant, bone, or mineral—it contains both stable and unstable isotopes of specific chemical elements. For radiocarbon dating, the stable isotope is carbon-12, while the unstable counterpart is carbon-14. By understanding the origins of these unstable isotopes and their proportions within the elemental makeup, we can determine the ratio of isotopes present at the time the sample was formed.
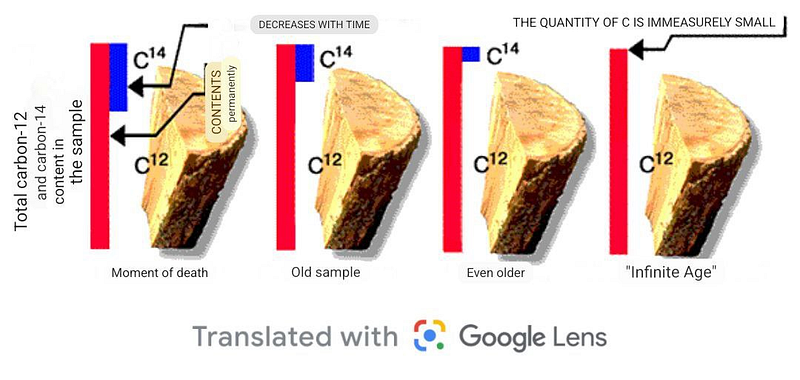
As the sample ages, no new atoms enter it; instead, the unstable isotopes gradually decay into different elements. By accurately knowing the half-life of the unstable isotope and measuring the current ratio of isotopes, we can ascertain the time elapsed since the object was created.
The first video, "Your Introduction to Isotopic Analysis," provides an overview of isotopic analysis principles and their significance in scientific research.
Chapter 1.2: Understanding Inaccuracies in Dating
As mentioned earlier, there is a kernel of truth in the criticisms surrounding the precision of radioisotope dating. A primary concern arises from uncertainties in the sample's history. For instance, if a sample has undergone melting, the dating may reflect the time after this melting event, or there may have been isotopic exchange with the environment. Thus, conducting a radiocarbon analysis on an item exposed to fire could yield a deceptively young age.

To mitigate these issues, scientists often trace the history of the sample, taking multiple samples of known ages for comparison. The most recognized dating method is radiocarbon dating, which can accurately date biological samples up to 60,000 years old with an accuracy of about 1%. This means that the younger the sample, the more precise the dating. The maximum potential error in this method is approximately 600 years, though often the error is much less. However, radiocarbon dating is not suitable for estimating the ages of fossils or rocks that are millions or billions of years old, for which other radioisotope methods must be employed.
The second video, "Stable Isotopes and the Food Web," explores how stable isotopes function within ecosystems and their applications in environmental science.
Chapter 1.3: The Calibration of Carbon-14 Levels
Additionally, radiocarbon dating is predicated on the assumption that atmospheric carbon-14 levels have remained constant over time, which is not necessarily accurate. Determining the actual concentration of carbon-14 during specific historical periods can be challenging.
To address variations in carbon-14 levels, calibration is done using tree rings. Each ring represents a single year of growth, allowing researchers to analyze wood from ancient trees to establish accurate carbon-14 concentrations for specific years, thereby enhancing the reliability of radiocarbon dating.
The significant jumps in carbon-14 levels identified in 774 and 994 AD, termed "Miyake events" after their discoverer, Fusa Miyake, were linked to intense solar flares. Research has revealed multiple Miyake events throughout history, which have significantly refined dating accuracy.
Chapter 2: Diverse Methods of Radioisotope Dating
Numerous radioisotope dating methods exist, each suited to specific age ranges and sample types. Here are some of the most commonly used and precise techniques:
- Uranium-lead method: Ideal for dating objects millions of years old, it can also apply to samples that are billions or thousands of years old with an accuracy of over 0.1%.
- Lead-lead method: This method is applicable to samples that have recently lost uranium due to environmental interactions, with a similar age range as the uranium-lead method.
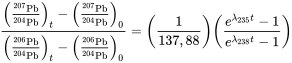
- Potassium-argon method: This technique is used for dating samples ranging from thousands to billions of years.
- Samarium-neodymium method: This method is useful for determining the age of rocks with precision up to 1 billion years.
If you’re interested in more articles about space, feel free to clap!
Consider subscribing to our channel and submit your questions for future articles.
If you appreciate my work, you can support me by becoming a member on Medium for just $5 a month, which helps us create even more engaging content.