Transgenic Mice: Pioneering Advances in Biomedical Research
Written on
Chapter 1: The Impact of Transgenic Techniques
The advent of genetic innovations such as fluorescent reporter genes, optogenetics, and DREADD has revolutionized the ability of scientists to modify the functions of specific cells in living organisms.
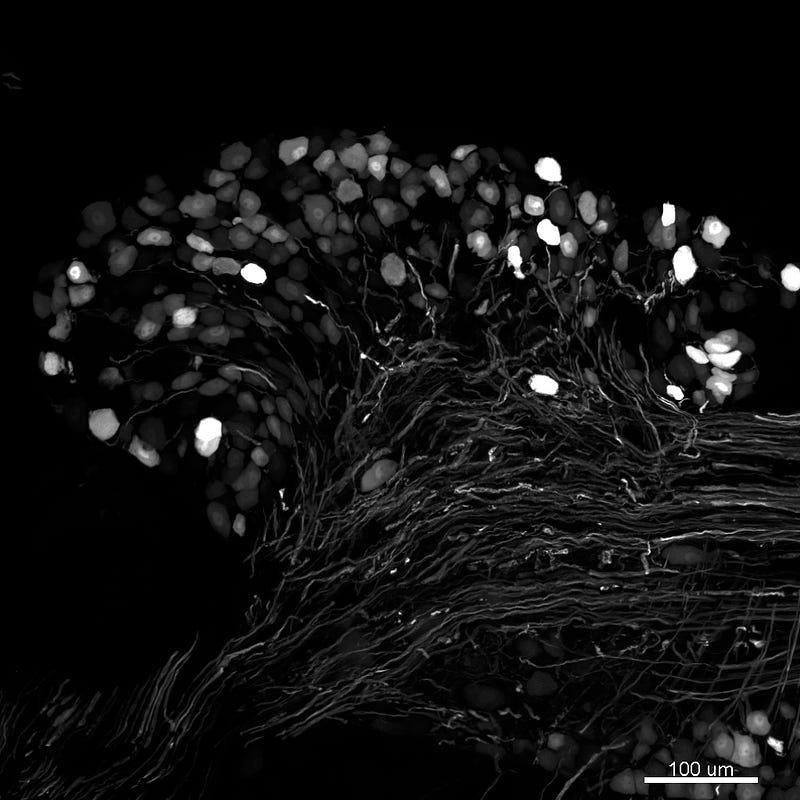
Figure 1. Sensory neurons and axons in the dorsal root ganglion of a mouse expressing green fluorescent protein (GFP). Confocal microscope image taken by the author.
A number of my scientific peers are enthusiastic about the emergence of transgenic methodologies. These advanced techniques in molecular biology involve transferring genes between species or engineering synthetic genes for expression in living organisms. They symbolize the culmination of extensive research across diverse fields, including molecular biology, protein chemistry, cellular biology, neuroscience, microbiology, virology, and animal behavior.
Looking ahead, it's anticipated that transgenic approaches will be adapted for human applications, potentially revolutionizing medicine and offering cures for currently untreatable diseases. Despite this promise, public awareness remains limited, likely due to the contentious nature of animal research and the political sensitivities surrounding genetic modification. Scientists often shy away from discussing their work, even though it represents one of modern science’s most extraordinary advancements.
Explaining these techniques can be complex, but let’s delve into it.
Section 1.1: Understanding Knock-In Genes
The methods I’m discussing are referred to as genetic knock-in techniques. Genes are sequences of DNA that translate into proteins, functioning as molecular machines that perform a vast array of cellular tasks. By altering a gene, we can modify the protein it produces, impacting the cell’s function, which ultimately manifests as a change in the organism.
Gene translation starts with a short DNA sequence called a promoter, located just upstream of the gene. Gene knockout involves manipulating a gene to render it non-functional, while gene knock-in entails making an organism express genes from another organism or synthetic genes that code for new proteins.
In biomedical research, knock-in techniques are primarily utilized in several animal species, including the nematode C. elegans, the fruit fly Drosophila, zebrafish, and mice. Mice are particularly relevant for human translation due to their physiological similarities to humans, especially in their nervous systems and genetic makeup.
The first video, "Probing the Brain Using New Transgenic Mice," discusses how these innovative models are being utilized to explore brain functions and disease mechanisms.
Subsection 1.1.1: Methods of Gene Insertion
Gene knock-in can be achieved through two fundamental approaches:
- Germ Line Insertion: In this method, new genes are integrated into reproductive cells, ensuring their presence in the offspring's germ line. Consequently, these genes are inherited by future generations but may not be expressed in all cells unless the appropriate promoter is activated.
- Somatic Line Insertion: This method restricts new genes to specific cells within an organism, typically using viral vectors that deliver genetic material directly to targeted cell types. Viral vectors are engineered to lack their original disease-causing genetic material, enabling them to infect cells without causing illness.
Transgenic methodologies often employ a combination of germ line and somatic line insertions for dual gene administration. Only the cells that encounter both genes will express the resultant protein, thereby altering their function.
Section 1.2: Ethical Considerations in Gene Editing
The ethical implications of germ line versus somatic line insertion are significant. While somatic line modifications are permissible in humans since they do not affect future generations, germ line changes pose risks of permanently altering the human genome, leading to ethical dilemmas similar to opening Pandora's Box.
A crucial aspect of these transgenic techniques is that they enable targeted expression of artificial proteins in specific cell populations, even amidst diverse cell types. This targeting leverages the unique gene expression profiles of various cell types.
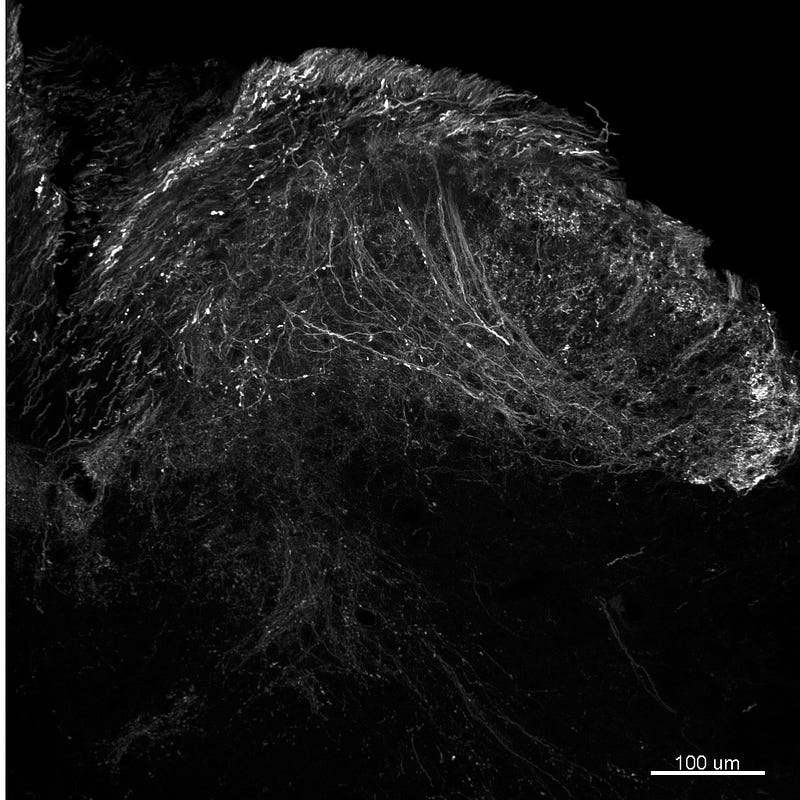
Figure 2. Sensory axons labeled with green fluorescent protein in the dorsal horn of the spinal cord of the same mouse as in Figure 1. Confocal microscope image taken by the author.
Chapter 2: Advancements in Fluorescent Proteins
The second video, "The Basics of Transgenic Mice: Pronuclear Injection," outlines the foundational techniques used to create transgenic mice, including the role of pronuclear injection in gene transfer.
Fluorescent proteins, derived from organisms like jellyfish and corals, emit light when exposed to specific wavelengths. Scientists have successfully incorporated these genes into the genomes of mice, enabling selective visualization of specific cell types based on their unique gene promoters.
This has led to groundbreaking advancements in physiology and anatomy, providing enhanced resolution in identifying and mapping various cell types. The initial use of green fluorescent protein (GFP) has given rise to a palette of fluorescent proteins, each named to reflect its color or origin, such as mCherry or Venus.
In my own research, I have utilized fluorescent proteins as reporter genes, which illuminate cells expressing specific transgenes, facilitating the identification and analysis of targeted cellular activities.
Humanized Mice and Their Implications
Opponents of animal research often argue that animal physiology diverges significantly from human physiology, which is misleading. While it’s true that minor variations in protein structures can affect drug efficacy across species, the underlying biological processes remain strikingly similar.
By replacing mouse genes with their human counterparts, scientists can enhance the predictive power of animal models for human drug responses. For instance, substituting the mouse mu-opioid receptor gene with the human version allows for accurate testing of opioid medications without generating hybrid organisms.
Conclusion: The Future of Transgenic Research
As transgenic technologies like DREADD and optogenetics continue to evolve, they hold the potential to fine-tune brain functions and provide novel therapies for various neurological disorders. However, ethical considerations surrounding germ line modifications must be carefully navigated.
Ultimately, transgenic research in animals plays an indispensable role in advancing biomedical science, allowing researchers to explore complex biological questions that in vitro methods cannot address. As we progress, it is crucial to maintain a balanced dialogue regarding the ethical implications and benefits of this vital research.
My historic romance novel Games of Love and Kink has just been published. You’ll love it!
Please visit my website Sex, Science & Spirit, where you can find my articles in English and Spanish, plus my talks, novels, bio scientific papers, and photos.
Copyright 2022 Hermes Solenzol.