Navigating the Complexities of the COVID-19 Booster Debate
Written on
Understanding the Booster Shot Debate
The discourse surrounding COVID-19 booster shots in the United States has transformed into a cacophony of unanswered scientific, political, and ethical inquiries.
This section aims to provide clarity amidst the confusion surrounding booster shots.
Section 1.1 The Upcoming FDA Review
Next week promises to bring some much-needed insight as the FDA's expert panel is scheduled to convene on Friday to discuss and potentially endorse Pfizer’s booster shot. The CDC will follow suit with its own panel meeting next week, likely issuing additional recommendations.
Pfizer has sought FDA approval for a third dose of its vaccine for individuals aged 16 and above. Meanwhile, Moderna has submitted its application, but it will be reviewed later than Pfizer's.
Currently, the US has permitted third doses for those with compromised immune systems, and several countries are administering boosters to high-risk populations.
Subsection 1.1.1 Expert Opinions on Boosters
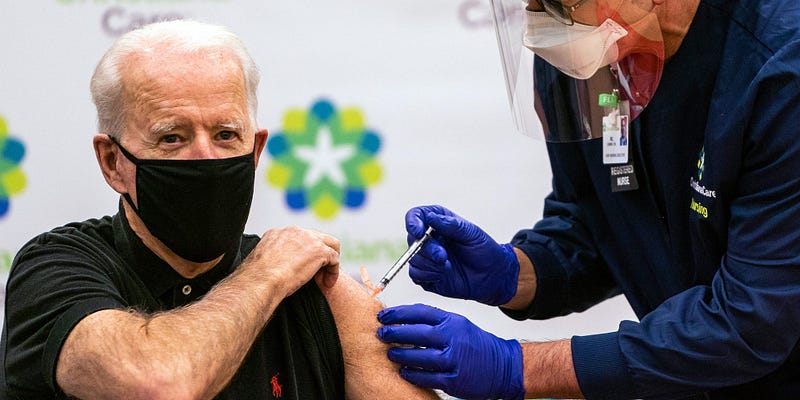
Experts are indicating that, for now, regulators may limit booster availability primarily to older individuals and the immunocompromised. As data continues to accumulate regarding younger populations, broader accessibility may become feasible if the advantages surpass any potential risks.
Dr. Megan Ranney, an emergency medicine professor at Brown University, expressed her expectation that boosters would eventually be administered to everyone, as is standard with vaccines. Dr. Larry Corey, a virologist at the Fred Hutchinson Cancer Center, shared his belief that offering boosters to individuals as young as 30 would be beneficial, albeit with caution regarding younger individuals due to concerns about myocarditis observed in the younger demographic post-vaccination.
“I strongly feel we’ll do more good by boosting than by watching,” Corey stated. “My nature is to act rather than simply monitor cases or fatalities.”
Section 1.2 The Biden Administration's Initial Announcement
The conversation surrounding booster shots gained momentum on August 18, when top public health officials from the Biden administration, including leaders from the FDA and CDC, issued a joint statement indicating that booster shots would soon be available for all Americans, starting September 20, for those eight months past their second dose. This announcement was made in the context of the Delta variant's rapid spread across the nation.
However, experts criticized this assertion as premature, suggesting it injected an unwanted political dimension into the scientific discussion. At that point, data regarding booster efficacy had not yet been submitted for FDA evaluation.
“The announcement created confusion by implying that a scientific consensus had already been reached,” noted Ranney.
Chapter 2 The Questions Surrounding Booster Efficacy
As experts consider the value of booster shots, especially for older individuals or those with weakened immune systems, the effectiveness of these doses remains uncertain. Questions linger about whether boosters will provide lasting protection or prevent breakthrough infections.
Dr. Jeremy Faust, an emergency medicine physician at Brigham and Women’s Hospital, emphasized the ambiguity surrounding vaccination objectives. Are vaccines intended to halt virus transmission and infections, or merely to reduce hospitalizations and mortality rates?
“The confusion stems from changing expectations regarding vaccine efficacy,” Faust explained, noting that initial assurances about vaccine performance have evolved over time.
Section 2.1 The Financial and Ethical Dimensions
The ongoing dialogue is further complicated by business and ethical considerations. Pfizer and Moderna have been vocal advocates for booster shots, but their financial interests in vaccine sales are substantial, with Pfizer anticipating over $33 billion and Moderna projecting $20 billion in 2021 revenue from their vaccines.
Ethical dilemmas also arise, particularly as the World Health Organization urges wealthier nations to postpone booster distributions until lower-income countries can secure initial vaccinations. However, affluent nations have largely disregarded these appeals.
Conclusion: A Path Forward
Despite the ongoing confusion, a middle-ground approach regarding booster shots appears to be the most plausible outcome. Experts advocate for making boosters available to those aged 60 and older, who face the greatest risks from COVID-19.
As for younger adults, the urgency is diminished, given that vaccines continue to provide effective protection against severe illness in this group.
Faust, who is 42, expressed his personal hesitance about receiving a booster, stating he would prefer to wait for more data before making a decision.
“I would feel uncomfortable getting a booster right now until I have more information,” he said, contrasting the elation he felt during his initial vaccination with the apprehension he feels now.
If you have a story regarding the coronavirus pandemic you wish to share, reach out to us at [email protected].