Innovative CRISPR Approach: Transforming Fat Cells to Combat Obesity
Written on
Chapter 1: Understanding Body Fat
Body fat exists in three distinct forms, each playing different roles in metabolism. The most common is white fat, characterized by large lipid droplets that serve as energy reserves. Most individuals possess an abundance of this fat type.
In contrast, brown fat comprises numerous smaller lipid droplets filled with mitochondria, which generate heat through energy expenditure (thermogenesis). Adults typically have minimal brown fat, located primarily in regions such as the shoulder blades, neck, spine, and kidneys. However, infants and certain hibernating animals have substantial amounts of this beneficial fat.
Lastly, there’s beige fat, a hybrid of white and brown fat that exhibits some thermogenic properties. While not identical to brown fat, beige fat contains more mitochondria than white fat and can be influenced by cold exposure and physical activity, potentially aiding in calorie burning and possibly protecting against cognitive decline.
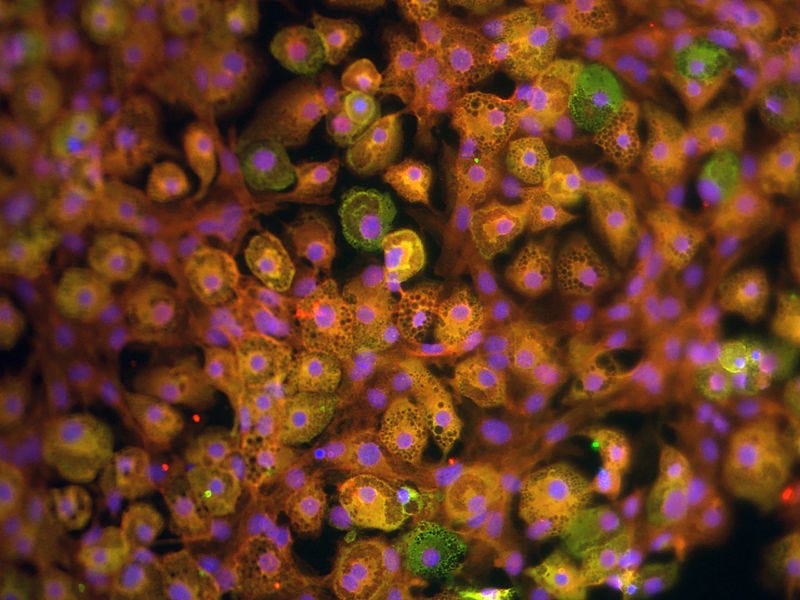
Chapter 2: CRISPR Technology and Fat Cell Engineering
Recent research has introduced a groundbreaking method to genetically modify white fat cells to mimic the behavior of brown fat. Scientists extracted a sample of human subcutaneous fat cells and employed CRISPR technology to alter the NRIP1 gene, which inhibits thermogenic activity in white fat.
By silencing this gene, the modified cells exhibited enhanced metabolism of glucose and fatty acids. The results were striking: when these CRISPR-modified cells were implanted into mice on a high-fat diet, there was a notable decrease in body fat and triglyceride levels, alongside improved glucose tolerance.
The first video, "CRISPR-Enhanced Adipocyte Browning: A Cell Therapy Approach to Treat Metabolic Disease," delves into the potential of CRISPR technology for transforming fat cells and improving metabolic health.
However, why not directly inject the CRISPR system instead of going through extraction and implantation? This approach allows for precise targeting of fat tissue. NRIP1 affects multiple genes across various tissues, so a blanket suppression could lead to unintended consequences. By working with extracted samples, researchers can also monitor for any off-target effects, which, in this study, were absent.
Interestingly, mice with suppressed NRIP1 remain lean and demonstrate resistance to obesity; however, this condition can lead to infertility in females, highlighting the importance of careful gene targeting.
It's essential to remember that findings from mouse studies may not fully translate to humans due to differences in metabolism and lifestyle. The researchers conclude that their work represents a promising therapeutic strategy for enhancing metabolic health through CRISPR-modified human adipocytes without introducing immunogenic responses.
The second video, "Joslin research: HUMBLE cells can improve metabolic health," explores how cellular modifications can enhance overall health and metabolic function.
A lingering question remains: even with enhanced mitochondria, can CRISPR-modified white fat cells rival brown fat in energy production? Future studies comparing these cell types could provide valuable insights.
If successful in humans, this approach could revolutionize weight loss strategies. Imagine undergoing liposuction, having the fat genetically modified, and subsequently enjoying a boost in metabolic rate and improved glucose management. While lifestyle changes are beneficial, the reality of daily life often limits the time available for such efforts. (Note: This is a humorous exaggeration.)